Opioid analgesics, such as morphine, are an essential medication which provide unparalleled pain relief in the acute setting, but are limited by their addictive liability and severe side effects, including respiratory depression.
In this project, we systematically assessed the pharmacology of opioid ligands, from cellular signalling assays through to profiling effects in vivo, including employing genetically modified animals.
The broader field, and most of this work, was described in a short narrative review.
Do distinct signalling pathways mediate opioid analgesia and side effects?
A significant field in opioid research, particularly on the µ-opioid receptor which is the primary target of current analgesics, has been investigating the signalling pathways which mediate the effects of these drugs.
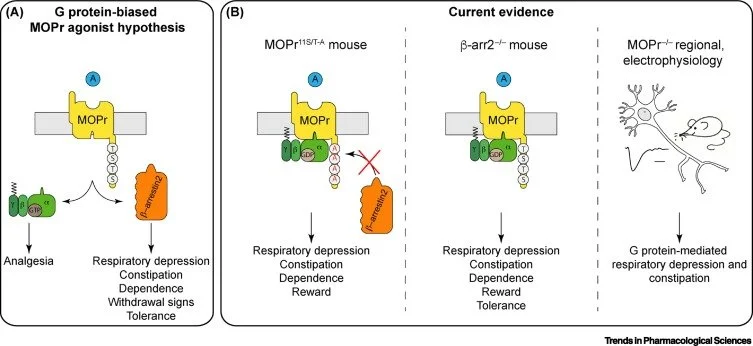
One hypothesis which has had particular interest is that opioid analgesia occurs through G protein-signalling, whereas side effects such as respiratory depression occurred through a proposed β-arrestin-dependent signal.
As part of a research consortium, we re-examined this hypothesis. We employed genetically-modified animals to test the contribution of β-arrestin to opioid effects, and found it was not required for deleterious opioid effects, in contrast to earlier results. See also Kliewer et al.
What are the challenges in quantifying receptor activity?
Based on what might be called the ‘β-arrestin2 hypothesis’, putatively G protein-biased compounds were developed, which would activate G protein-signalling without recruiting β-arrestin to the receptor.
Key confounds are however often present in assays of receptor activity - including ‘floor’ effects due to low sensitivity, 'ceiling’ effects due to limitations in reporter assays, and kinetic limitations.
We found all of these confounds had affected characterisation of putatively G protein-biased compounds, and, when addressed, these ligands were not biased and would be better described as simply low efficacy agonists.
This work was a collaboration between several research groups. In a separate paper, we analysed how such confounds led to this mischaracterisation.
What properties of opioid agonists contribute to their in vivo profile?
Opioid agonists, including both existing drugs and new compounds differ in many crucial respects - affinity for the receptor, the kinetics of that interaction, and the efficacy of a compound for activating the receptor.
Putatively G protein-biased compounds with low efficacy had been described as having improved side-effect profiles, particularly in regard to respiratory depression.
By more accurately quantifying agonist efficacy, and by comparing new compounds to a broader panel of existing drugs, we were able to observe that the low intrinsic efficacy of this proposed new class of drugs explained the slightly improved in vivo profile.